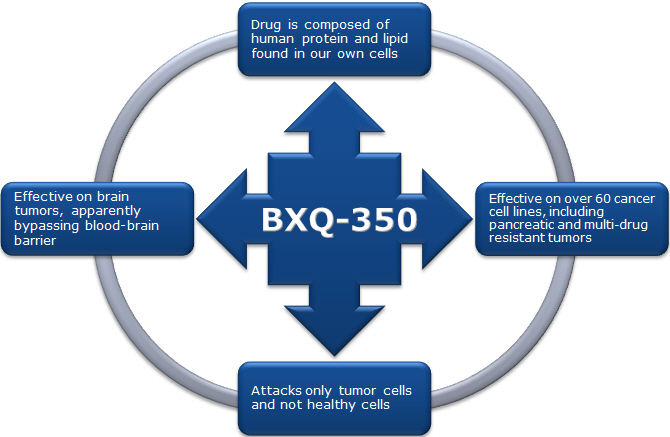
COVINGTON, Ky. — Bexion Pharmaceuticals Inc. announced Tuesday Part 3 of their Phase I First-In-Human Trial using BXQ-350 for the treatment of cancer has exceeded expectations in screening and enrollment at their four trial sites.
In the first few weeks of opening Part 3, over 20 GI and other solid tumor patients enrolled with additional patients in screening. The purpose of Part 3 is to explore safety and additional indications in rare and gastrointestinal tumors.
The Phase I Part 1 study showed that BXQ-350 was well tolerated at all five doses tested with no dose limiting toxicities observed and with no serious adverse events attributed to the therapy. Part 2 tested the highest dose in an additional 36 solid tumor patients. Preliminary data support a safe and tolerable drug profile.
“The safety profile of our drug illustrated in Phase I Part 1 and 2 of our trial has created enthusiasm about BXQ-350 among our principal investigators,” stated Dr. Ray Takigiku, founder and CEO of Bexion. “The objective of Part 3 is to build and expand on the impressive results observed in our prior Phase 1 studies. The rapid enrollment is a reflection of the strong safety profile demonstrated for BXQ-350 and underscores the unmet medical need for new and novel treatment options in these patient populations.”
• Cincinnati.com: Patient No. 1 and the cancer trial here that just might change everything




















Add Comment